The chemists from Utrecht University have discovered a way to make the solid substance lithium borohydride (LiBH4) more than 1,000 times as conductive at room temperature. This discovery was made by chance as part of their research into materials that can reversibly store hydrogen. One of the materials they studied was LiBH4, which was enclosed in the nanometres-sized pores of a silica sponge.
This discovery leads to new opportunities for innovation. The current generation of batteries contain organic fluid that conduct ions in lithium batteries. Replacing the organic fluid by a nanocrystalline sponge could make the new batteries more compact, sustainable and safe.
More specifically, the layman’s abstract of the article describes:
“Charging and discharging a battery implies that charged species (ions) need to shuttle rapidly from one side of the battery to the other. At room temperature ions can move fastly through liquids, but generally only very slowly through solids. Hence today’s lithium-ion batteries contain liquids, while avoiding them might lead to safer, more durable, and lighter batteries. We surprisingly found that if a lithium-ion containing solid material is confined to the very small pores of a silica scaffold, lithium-ion movement in the solid becomes more than two orders of magnitude faster at room temperature. Our results point to a new strategy to design all-solid state batteries based on fast ion conducting solids.”
Note: the Utrecht chemists developed the material, the lithium mobility was measured in Nijmegen, while the Danish researchers performed the conductivity experiments.
The full press release about the discovery can be read on the website of Utrecht University.
Read the full article in Octobers edition of Advanced Functional Materials.
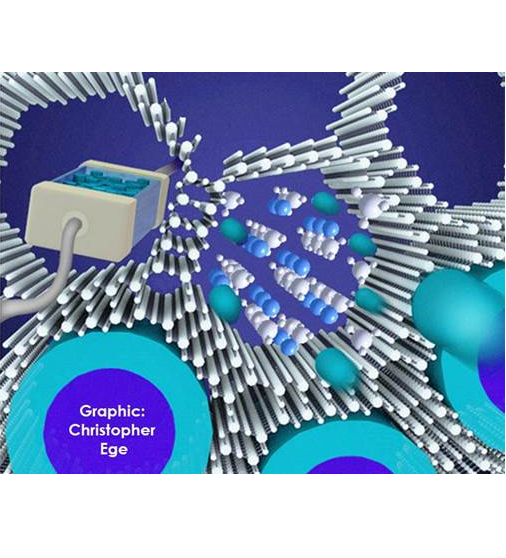
This drawing depicts lithium-ions (blue balls) moving fast in a nanosilica scaffold.
