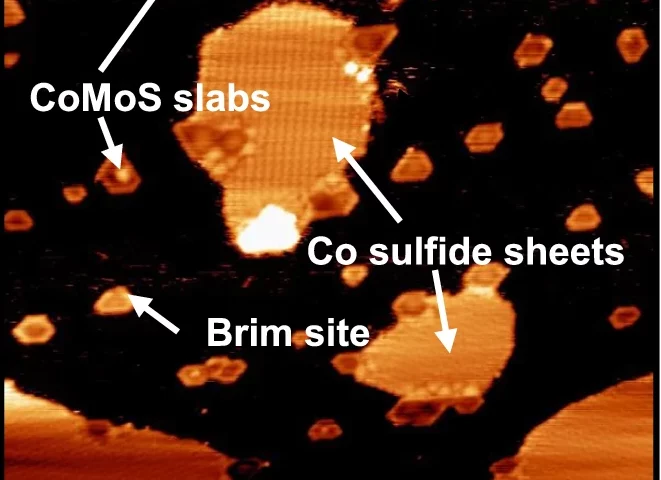
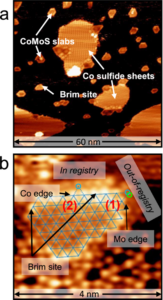
Sulfur is an undesirable impurity in many feedstocks for the production of transportation fuels and chemicals, both fossil-based and biomass-based. While the process of hydrodesulfurization is one of the largest industrial catalytic processes, many details of the exact mechanism, and especially the role of promotors in the catalytic process are still under debate. In collaboration with Leiden University and Ketjen Research, Utrecht researchers have now studied the reaction in high pressure in situ scanning tunneling microscopy, and used density functional theory calcula
tions to resolve the atomic structures and reaction pathways. The work shows that Co-promotors in the catalyst stabilize the adsorbed reactant molecules, thus enhancing the probability of HDS to occur, and suggests a new pathway involving methyl transfer on the Co-promoted edges of MoS2 catalysts. The density functional theoretical results closely match the observed structures of intermediates at the catalytic surface.
Article:
M.K. Prabhu1, J.N. Louwen2, E.T.C. Vogt3 & I.M.N. Groot1. Hydrodesulfurization of methanethiol over Co-promoted MoS2 model catalysts, Nat Commun 15, 7170 (2024).
https://doi.org/10.1038/s41467-024-51549-6
1 Leiden University
2 Ketjen Research
3 Inorganic Chemistry and Catalysis group, Utrecht University